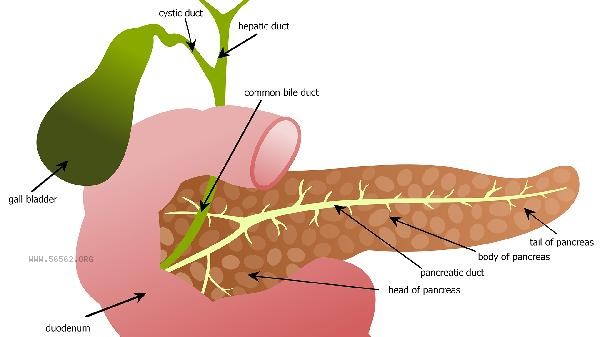
The detection of cholesterol esterase activity is mainly achieved through five methods: spectrophotometry, fluorescence, isotope labeling, high-performance liquid chromatography, and electrochemical methods. The detection results are affected by factors such as sample type, reaction temperature, substrate concentration, pH value, and inhibitor.
1. spectrophotometric method:
calculates enzyme activity by measuring the absorbance changes of free cholesterol produced by the hydrolysis of cholesterol esters in the reaction system at specific wavelengths. The commonly used 4-aminoantipyrine reacts with peroxidase to generate a red quinone imine compound, and the absorbance is detected at 500nm. This method is easy to operate but is susceptible to interference from hemolysis and jaundice samples.
2. Fluorescence method:
uses fluorescently labeled cholesterol analogues as substrates, and the intensity of fluorescent groups released after enzymatic hydrolysis is proportional to enzyme activity. The commonly used NBD labeled cholesterol 22-N-7-nitrobenzene-2-oxa-1,3-diazol-4-ylamino-23,24-didemethyl-5-cholestene-3 β - ol has a detection sensitivity 10-100 times higher than spectrophotometry and is suitable for trace sample detection.
3. Isotope labeling method:
uses radiolabeled cholesterol esters such as [3H] cholesterol oleate as substrates, and measures the amount of free [3H] cholesterol released after enzymatic hydrolysis using a liquid scintillation counter. This method has strong specificity but poses a risk of radioactive contamination and requires operation in a professional laboratory.
4. High performance liquid chromatography:
separates and quantifies the reaction products cholesterol and fatty acids by HPLC, and can simultaneously determine multiple lipid components. Using a C18 reverse phase chromatography column and a UV detector at 205nm, it can accurately distinguish between cholesterol ester hydrolysis products and unreacted substrates.
5. Electrochemical method:
is based on the coupling reaction between cholesterol oxidase and cholesterol esterase to determine the hydrogen peroxide current signal produced by enzymatic hydrolysis. Using a platinum electrode for detection at+0.6V voltage has a fast response time and is suitable for continuous monitoring of enzyme kinetics changes.

Venous blood should be collected on an empty stomach for 12 hours before testing to avoid vigorous exercise affecting blood lipid levels. Serum samples should be stored at 4 ℃ for no more than 72 hours, and long-term storage requires freezing at -80 ℃. Normal cholesterol metabolism can be maintained in daily life by controlling saturated fatty acid intake, increasing dietary fiber, and regular aerobic exercise. For those with abnormal detection, it is recommended to undergo follow-up and complete complementary examinations such as apolipoprotein A1 and B100. If necessary, genetic sequencing should be performed to rule out familial hypercholesterolemia.









Comments (0)
Leave a Comment
No comments yet
Be the first to share your thoughts!