COVID-19 vaccine of Sinopharm Group is about to be launched, and many people expect that this is the first COVID-19 vaccine in China, which means that all people can inject COVID-19 vaccine after it is launched.
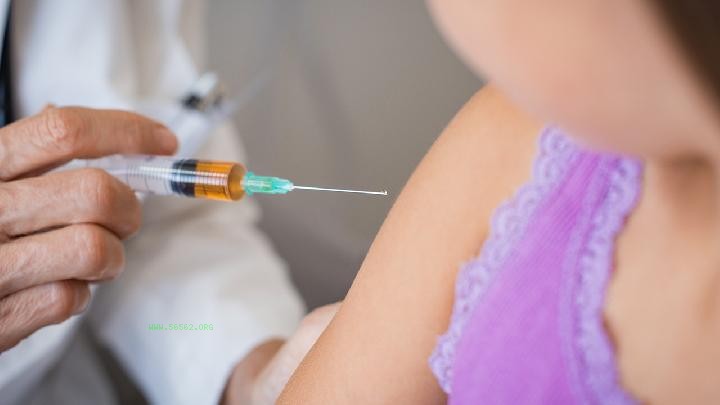
So, is Sinopharm COVID-19 vaccine reliable? Let's take a look at the introduction provided by our website together! Is the COVID-19 vaccine of Sinopharm reliable? Look at the popular science video of the vaccine. You should know that besides the effectiveness of the vaccine, there is also persistence, toxic side effects and other important data that need to be observed and waited. This all needs time to verify. If the vaccine comes out too quickly, the data is incomplete and risky. If it is still relatively safe in China, you should wait for the country to arrange it. - Is the COVID-19 vaccine of Sinopharm on the market? The latest news, Shi Shengyi, deputy general manager of Sinopharm, said that Sinopharm had submitted the COVID-19 vaccine listing application to the State Food and Drug administration.
This indicates that COVID-19 Vaccine of Sinopharm Group will be launched soon, and it is expected to be at the end of this year or early next year.
There is no comparison between Pfizer's COVID-19 vaccine and Sinopharm's vaccine before the release of phase III clinical trial results. To be frank, vaccines without phase three results are not eligible for comparison with vaccines with phase three results.
Antibody titer was measured in Phase II clinical test, but the antibody titer was only a soft endpoint, while the incidence rate in Phase III clinical test was the hard endpoint.
Vaccines with high antibody titer may, but not necessarily, significantly reduce the incidence rate.
It's like, whether a person's martial arts skills are high or not cannot be judged solely by playing a set of swordsmanship, but by their actual combat performance.
Of course, if the antibody titer is low, it's like not being able to play swordsmanship tricks efficiently, so there's a high probability that the actual combat strength won't be very good.
For other vaccines in the past, placebo controlled phase III clinical trials will be conducted for the first product, just like the phase III trials of various COVID-19 vaccines.
But once the effectiveness of the first product is confirmed (reducing the incidence rate), for ethical reasons, other similar products will not do the placebo control test, otherwise it is too cruel to vaccinate people with placebo, and we will compare the first product that has been verified. In summary, there is no comparison right now, we have to wait until the results of the Phase III clinical trial of the Sinopharm vaccine are available.







Comments (0)
Leave a Comment
No comments yet
Be the first to share your thoughts!